Cleaning, Disinfecting, and Sterilizing the Probe
All probes must be cleaned and disinfected after each use. Cleaning is an important procedure that must be
carried out before disinfecting the probe. For information on cleaning and probe disinfection, please
refer to ‘Cleaning, Disinfecting, and Sterilizing the Probe’ in the ‘Probes’ chapter of the user manual.
Using an inappropriate disinfectant may damage the probe.
-
 WARNING
WARNING
-
- Always use protective equipment such as face mask, eyewear, and gloves when cleaning,
disinfecting, and sterilizing probes.
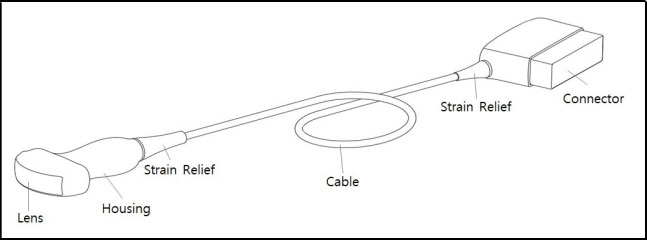
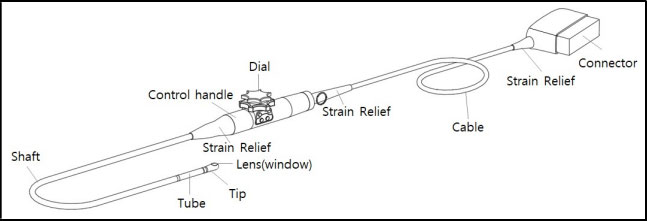
- Inspect the housing, strain relief, lens and seal for damage, and check for any functional
degradation after cleaning and disinfecting the probe.
- Using an inappropriate cleaning or disinfecting agent may damage the probe.
Information on Detergent, Disinfectant, and Ultrasound Gel
Reprocessing Method by Probe Type
Ultrasound probes are classified into Critical, Semi-critical or Non-critical devices based on the standards of FDA
guidance* and the Hygiene Requirements for the Reprocessing of Medical Devices from Germany guideline of Robert Koch
Institute (RKI). Therefore, you should use the cleaning, disinfection, and sterilization methods appropriate for
each
classification. Proper maintenance is also required to maintain the performance of ultrasound probes.
Choose the Correct Probe-Care Method in the Below Table
| Classification Criteria |
Contact Area |
Application Probe |
Level Selection |
| Non-critical devices |
Intact skin |
Curved, Linear, and Phased array probes |
Low level disinfection |
| Semi-critical devices |
Mucous membrane, damaged skin |
Endocavity, MPTEE |
High level disinfection or sterilization |
| Critical devices |
Blood, sterile tissue, etc. |
Intraoperative |
Sterilization |
* Guidance for Industry and FDA Staff – Marketing Clearance of Diagnostic Ultrasound Systems and
Transducers - Appendix E
* The FDA reprocessing guidance ‘Reprocessing Medical Devices in Health Care Settings: Validation Methods
and Labeling, Guidance for Industry and Food and Drug Administration Staff’ March 17, 2015,
(https://www.fda.gov/media/80265/download)
The care method for your probes determines the appropriate disinfectant to use for your probe. An appropriate
detergent, disinfectant or ultrasound gel should be used for all probes. For details about compatible
detergent, disinfectants, and ultrasound gel, please see ‘Disinfectants Matrix’ on Samsung Medison website
and the User Guide.
※ All disinfection methods for Semi-critical probes that are marked with ◆ in the attached Excel file have been validated by Samsung Medison for biological effectiveness.
- User Guide: This is provided as a booklet upon purchase of the product.
- Disinfectants_Matrix List: Please refer to the attached Excel file.
 ▲ Scan or click the QR code to visit 三星医疗
The personal information of existing Samsunghealthcare.com chinese users will be kept until October 29th and
will be safely deleted thereafter.
▲ Scan or click the QR code to visit 三星医疗
The personal information of existing Samsunghealthcare.com chinese users will be kept until October 29th and
will be safely deleted thereafter.
 ▲ Scan or click the QR code to visit 三星医疗
The personal information of existing Samsunghealthcare.com chinese users will be kept until October 29th and
will be safely deleted thereafter.
▲ Scan or click the QR code to visit 三星医疗
The personal information of existing Samsunghealthcare.com chinese users will be kept until October 29th and
will be safely deleted thereafter.


 CAUTION
CAUTION NOTE
NOTE
 WARNING
WARNING NOTE
NOTE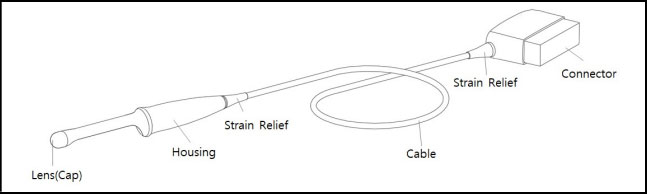
 WARNING
WARNING CAUTION
CAUTION




![불량 현상 - External damage, Drop out at the damaged area [외관 손상 및 이미지 줄빠짐] / 원인 - FPCB disconnection, Protrusion, Damaged part [충격(낙하)에 의한 내부 부품 파손] / 권장 가이드 - [지정된 홀더에 보관], [보호 Cover 장착]](/resources/kr/_img/sub/disinfection/warning_image/warning_tab5-1.jpg)
![불량 현상 [lens 부위 찍힘] / 원인 [Gel 토출구 및 날카로운 도구에 의한 찍힘], [Lens면에 Biopsy 체결부와 간석] / 권장 가이드 - Gel adapter [Lens와 간섭이 없도록 사용/Gel adapter 사용], [Biopsy 체결부위 확인 후 사용]](/resources/kr/_img/sub/disinfection/warning_image/warning_tab5-2.jpg)
![불량 현상 [Lens 마모] / 원인 [거친 Wipe 사용] / 권장 가이드 [부드럽고 보풀이 없는 Wipe/천 사용]](/resources/kr/_img/sub/disinfection/warning_image/warning_tab5-3.jpg)
![불량 현상 [Lens 들뜸] / 원인 - [비권장 소독제], [거친 타올 사용], [부적절한 소독기 사용], [Cover의 장시간 사용] - [부적절한 소독/사용환경] / 권장 가이드 - [권장 소독제], [부드러운 티슈 사용], [권장된 방법으로 소독기 거치], [Cover는 사용 후 매번 교체] - [권장소독제 및 부드러운 티슈 사용]](/resources/kr/_img/sub/disinfection/warning_image/warning_tab5-4.jpg)
![불량 현상 - Discoloration, Discoloration, Discoloration [Lens 및 Cable 변색] / 원인 - [요오드], Probe Cover [Cover 장시간 사용으로 소독제 노출] → 변색 - [비권장 소독제 사용] / 권장 가이드 - [Cover 사용 후 매번 교체] → [물 or 물티슈로 세척] → [티슈로 물기 제거 및 건조] - [Cover 매번 교체 및 세척]](/resources/kr/_img/sub/disinfection/warning_image/warning_tab5-5.jpg)
![불량 현상 [Cable 및 Switch box 손상] / 원인 [바퀴에 Cable 눌림 및 과도한 Bending], [비스듬한 장착] / 권장 가이드 [Probe 분리 후 시스템 이동], [과도한 Bending 지양], [체결 시 수평 확인]](/resources/kr/_img/sub/disinfection/warning_image/warning_tab5-6.jpg)
![불량 현상 [시술], [Sheath 제거] → [부드러운 Wipe로 Gel 제거] → [Cleaning] → [소독] → [권고 : Cover 장착 후 보관]](/resources/kr/_img/sub/disinfection/warning_image/warning_tab5-11.jpg)
![권장 가이드 [챔버 벽면에 닿지 않게, 수직으로 장착], [라인선 위로 Probe 장착] / 불량 현상 [충격에 의한 파손 발생]](/resources/kr/_img/sub/disinfection/warning_image/warning_tab5-7.jpg)
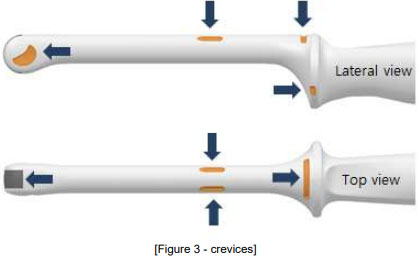
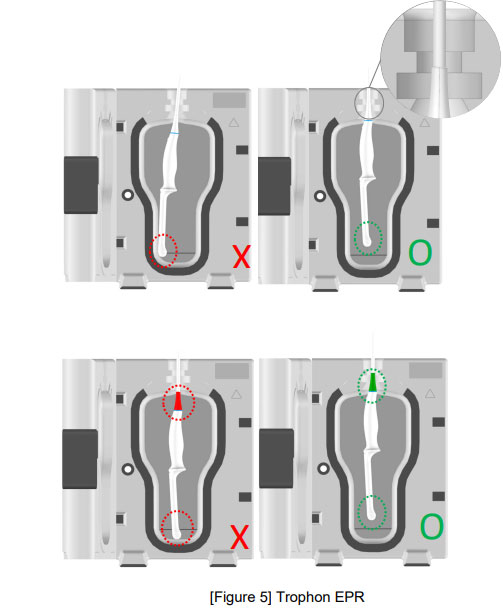
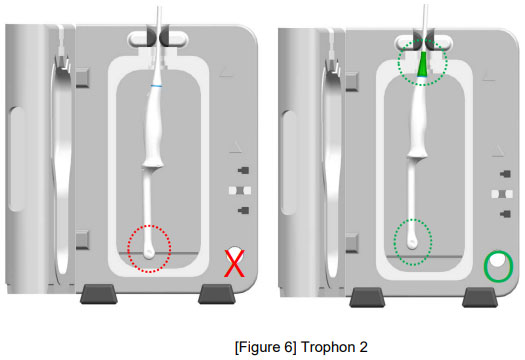
![권장 가이드 - Strain relief, Clamp [Strain relief 부위에 Probe 장착] / 불량 현상 [Strain Relief 틈새로 소독제 침투], [Strain relief 파손]](/resources/kr/_img/sub/disinfection/warning_image/warning_tab5-8.jpg)
![권장 가이드 - Non-contact, Non-contact, 벽면으로부터 최소 약 12cm 이상 떨어져 장착, 바닥면으로부터 최소 약 7cm 이상 떨어져 장착 [Probe 장착 시 챔버 내벽과 바닥에 닿지 않도록 거리 유지하여 장착]](/resources/kr/_img/sub/disinfection/warning_image/warning_tab5-9.jpg)
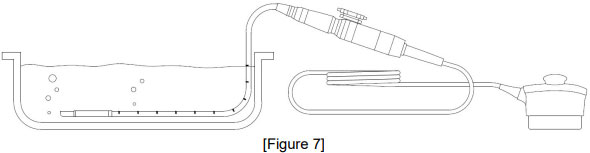
![권장 가이드 [1 Cycle : 90 Sec], 42℃ ↓ - [챔버 내 온도 : 42℃] / 불량 현상 - Transducer Membrane → 잔주름 발생, 황변](/resources/kr/_img/sub/disinfection/warning_image/warning_tab5-10.jpg)
